Abstract
Background: Although guidelines include direct-acting oral anticoagulants (DOACs) as an alternative to low molecular weight heparins (LMWHs) for treatment of cancer associated venous thrombosis (CAT), specific recommendations for selection of patients for DOAC treatment vary. Recommendations for use of DOACs are driven predominantly by perceptions of risk: benefit ratio (often associated with different cancer subtypes). We sought to assess patient characteristics and temporal changes in DOAC (vs. LMWH) utilization in patients being treated in routine practice for CAT.
Methods: This analysis is part of the Observational Studies in Cancer Associated Thrombosis for Rivaroxaban - United States (OSCAR-US) program. OSCAR-US is an ongoing study utilizing longitudinal patient-level medical record data from Optum for 91+ million patients seen at 700+ hospitals and 7,000+ clinics across the US. To be included in the present study, adult patients had to be diagnosed with active (primary or metastatic) cancer, undergone hospitalization, emergency department or observation unit admission associated with a primary International Classification of Diseases (ICD)-9th or -10 th revision diagnosis code for venous thromboembolism (VTE) between January 2013 and September 2020, received a DOAC or LMWH on day 7 of CAT treatment, and been active in the data set for at least 12 months prior to the CAT. Cohort assignment was based upon anticoagulant received on day 7 to increase the study's likelihood of appropriately classifying patients into their intended long-term treatment group. We defined active cancer as any cancer associated with ongoing treatment with chemotherapy, immunotherapy, radiation, or recent surgery; the presence of metastatic disease (regardless of time from initial cancer diagnosis); or provider encounter with a primary diagnosis code for cancer within the 6 months prior to the CAT. We excluded patients with an alternative indication for anticoagulation use or evidence of anticoagulation during the 12 months prior (per written prescription or patient self-report). For this analysis, year of CAT diagnosis was grouped into three mutually exclusive categories (2013-2015, 2016-2018, and 2019-2020) to represent early DOAC availability for VTE, peri-DOAC CAT trials and post-CAT guideline recommendation periods. Categorical data were reported as percentages, while continuous data were reported as means ± standard deviations (SDs). Chi-square and independent sample t-tests were used to test for differences in characteristics and treatment patterns between cohorts.
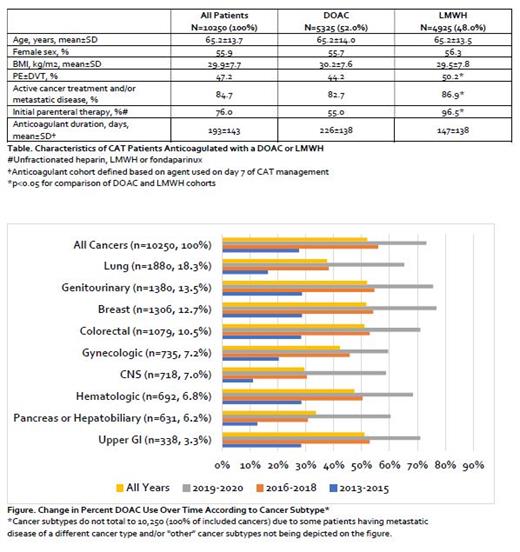
Results: In total, 95,072 adult patients experiencing a VTE-related hospitalization, emergency department or observation unit visit were identified. Of these, 14,377 (15.1%) met our pre-specified criteria for active cancer; including 84.7% who received a cancer treatment modality within 6-months and/or had metastatic disease (Table). Though a majority (76.0%) of patients received parenteral therapy as their first anticoagulant upon CAT diagnosis; on day 7 of CAT management, 5325 (52.0%) were receiving a DOAC and 4925 (48.0%) a LMWH. Mean age of patients was 65.2±13.7 years, body mass index (BMI) was 29.9±7.7 kg/m2 and 55.9% were women. Pulmonary embolism (PE)±deep vein thrombosis (DVT) was present in 47.2% of patients and more frequent in patients treated with LMWH than a DOAC on day 7 (p<0.001). The most common cancer types were lung (18.3%) genitourinary (13.5%), breast (12.7%) and colorectal (10.5%) (Figure). The proportion of patients treated with a DOAC increased substantially for all cancers over the three time periods evaluated (27.5% to 55.9% to 73.2%, p<0.001). This temporal relationship of increasing DOAC use (vs. LMWH) remained consistent across individual cancer subtypes (p<0.001 for all). Mean anticoagulant treatment duration was 193±143 days for the entire cohort and was longer in patients treated with DOACs vs. LMWHs (226±138 vs. 147±138 days, p<0.001).
Conclusions: In this US population, DOACs were increasingly being utilized for the management of CAT patients and for longer treatment durations than LMWHs. The finding of increasing frequency in use of DOACs vs. LMWHs appeared consistent across all major cancer subtypes. Given their common use, future analyses should evaluate the real-world effectiveness and safety of DOACs compared to LMWHs within individual cancer subtypes.
Coleman: Bayer AG: Consultancy, Honoraria, Research Funding; Janssen Scientific Affairs LLC: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals: Research Funding. Abdelgawwad: Bayer AG: Current Employment. Psaroudakis: Bayer AG: Current Employment. Fatoba: Bayer AG: Current Employment. Rivera: Bayer AG: Current Employment. Brobert: Bayer AG: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal